About Oral Pharmaceuticals
There are numerous oral preparations taken by mouth, with representative types including tablets, capsules, granules, and powders. Tablets, in particular, can come in forms such as uncoated tablets, film-coated tablets, and sugar-coated tablets. The primary packaging, which directly contacts with the drug, functions to maintain the quality of the drug and ensure user convenience. This primary packaging includes PTP (Press Through Package) blister packaging, strip packaging, sachet packaging, stick packaging, and bottle packaging. Primary packaging products are further wrapped with single or multiple layers of film or paper, carrying regulatory-required labels (such as batch number, expiration date, GS1 barcodes, etc.) to maintain the quality of the product during transportation and prevent errors and ensure convenience during usage.
Example of Manufacturing Process for Oral Pharmaceuticals
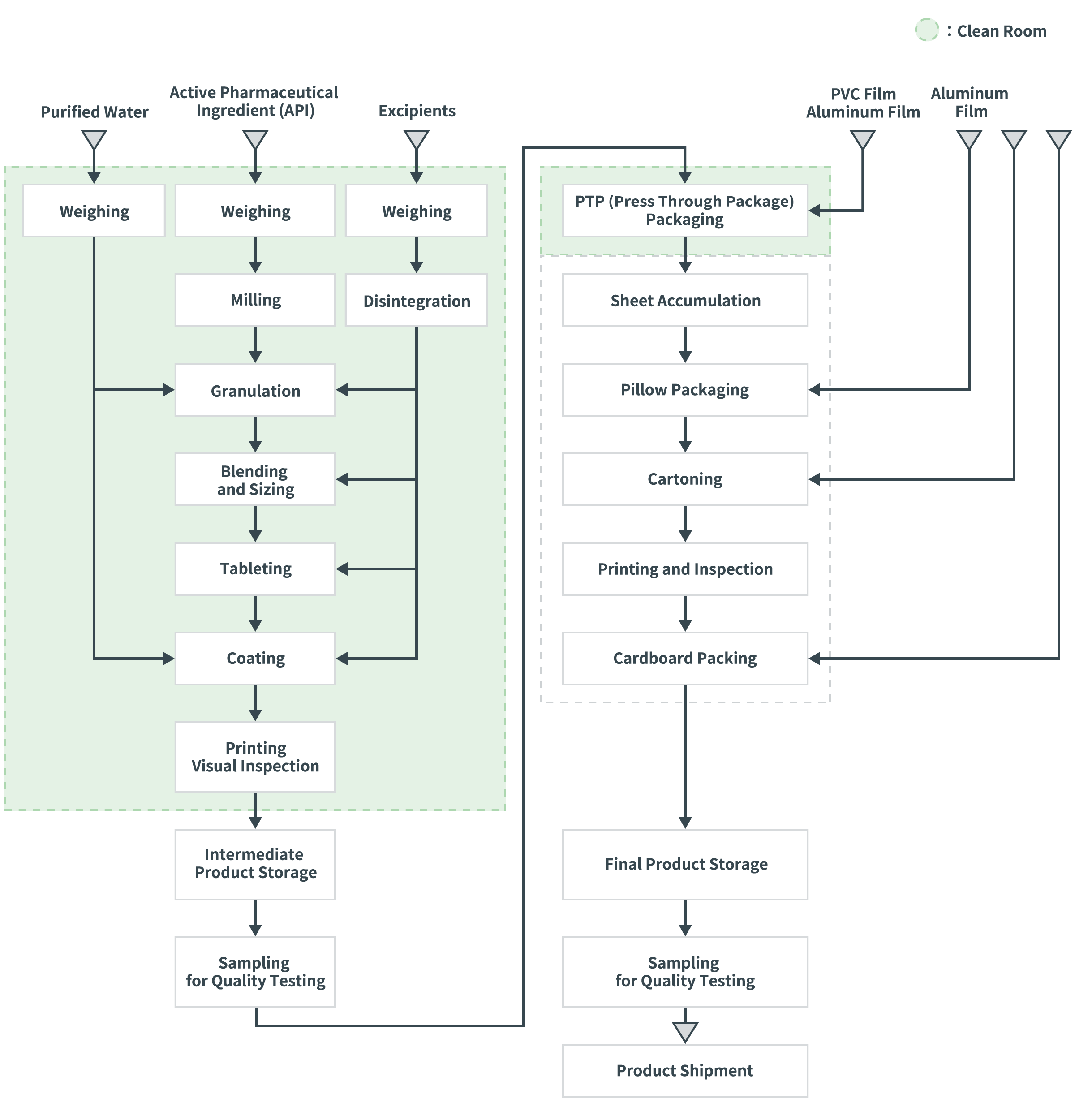
The following diagram illustrates a process flow chart for a film-coated tablet blister package product as an example of an oral formulation.
Process Flow Chart Example: Film-Coated Tablet Blister Packaging
- The preparation process and primary packaging process are conducted in a clean room where temperature, humidity, and particulate matter are controlled to prevent microbial and foreign matter contamination into the products.
- During the preparation process, raw materials and intermediate products for each batch are handled using containers between different process stages.
- Tablets are engraved or printed with the product name or other information. After they are visually inspected, samples are taken for quality testing.
- Lots that pass the quality tests proceed to primary PTP packaging and secondary packaging as pillow packaging, cartoning, printing & inspection and cardboard packing. Unlike the preparation process, where between each stage requires manual handling, the packaging process is normally fully automated. Lots that pass the final quality tests are shipped.
Key Points of Oral Pharmaceutical Manufacturing Equipment
Handling of High Potency Pharmaceuticals
With the increase in high potency pharmaceuticals such as anticancer drugs, containment performance using isolators and split valves is required for equipment handling particularly dispersive powder raw materials, from the perspective of both product quality and operator safety.
Batch Manufacturing and Equipment Integration
In preparation processes, each batch is handled using containers (with different designs for powders and tablets). This requires design considerations for the integration of lifter or vacuum transfer equipment to manufacturing equipment.
Design of Preparation Equipment Required for Containment
For product requiring containment, split valves are used, necessitating high-precision alignment of equipment horizontally and vertically. This involves complex design considerations including container and valve operation.
Evaluation and Selection of Production Equipment

Production equipment includes mills, granulators, tablet presses, film coaters, capsule filling machines, weight sorting machines, and packaging machines, are evaluated and selected from containment, capabilities, sampling methods, dust collection, cleaning and other various points of view from domestic and international manufacturers. Containers washing machine also required.
Mechanical Flow Diagram

Considering these factors, a mechanical flow diagram is created and reviewed with customers during the design phase.
Continuous Manufacturing
In place of conventional batch methods using containers, continuous manufacturing equipment automatically transfers raw materials and intermediates between processes is recently developed. Technologies such as Process Analytical Technology (PAT) inline, online, and at-line using NIR sensors or Raman spectroscopy ensure direct product quality monitoring, offering potential for real-time release testing (RTRT).
Tablet Engraving and Printing
Tablets are used to be engraved during tableting for identification. To improve identification, product names in katakana or other identifiers can be printed directly onto tablets using inkjet or laser printing, with many options available from various mahnufacturers to suit specific needs.
Prevention of Medical Errors and Traceability
To prevent medical errors from drug mix-ups and ensure traceability, barcode labeling has been mandated in packaging processes, requiring printing and inspection equipment. For exports, compliance with serialization and other regulations of the target countries is also necessary.
Anti-Counterfeit Technologies
High-cost pharmaceuticals, especially for export, employ various anti-counterfeit technologies in labeling materials.
Contamination Prevention Measures

To prevent cross-contamination of different products, production spaces should be zoned and laid out to facilitate line clearance at the start and end of production. As inspection equipment increases, the number of rejected items rises. Minimizing false positives and planning adequate space for handling rejected items are critical to reducing the risk of contamination. Shared washing areas for re-cleaning containers and considerations for residual checks within product cans during transport are also important.
Strengths of CM Plus
①Engineering + CM Method
The CM method is a project management system that integrates the client’s perspective with designers to operate and manage projects transparently, ensuring success from the aspects of QCD (Quality, Cost, Delivery) + EHS (Environment, Health, Safety). Professionals, including Construction Managers (CMr) who specialize in management, work on behalf of the client.

At CM Plus, we conduct engineering (design) in-house. We have engineers who are well-versed in manufacturing processes, equipment and facilities, construction, and manufacturing support facilities (such as electrical systems, HVAC, and utilities). This allows us to oversee the entire facility from the initial planning stages, enabling the construction of integrated and cohesive layout facilities.
②Comprehensive Coordination of Production Equipment and Building Facilities
With a focus on designing from the production process perspective, we execute designs that start from the internal aspects like building production equipment lines, layouts, and internal flow/logistics, leading to the creation of API (Active Pharmaceutical Ingredient) factories that are in harmony with the production system. Our planning extends to the intangible aspects of the production system as well.
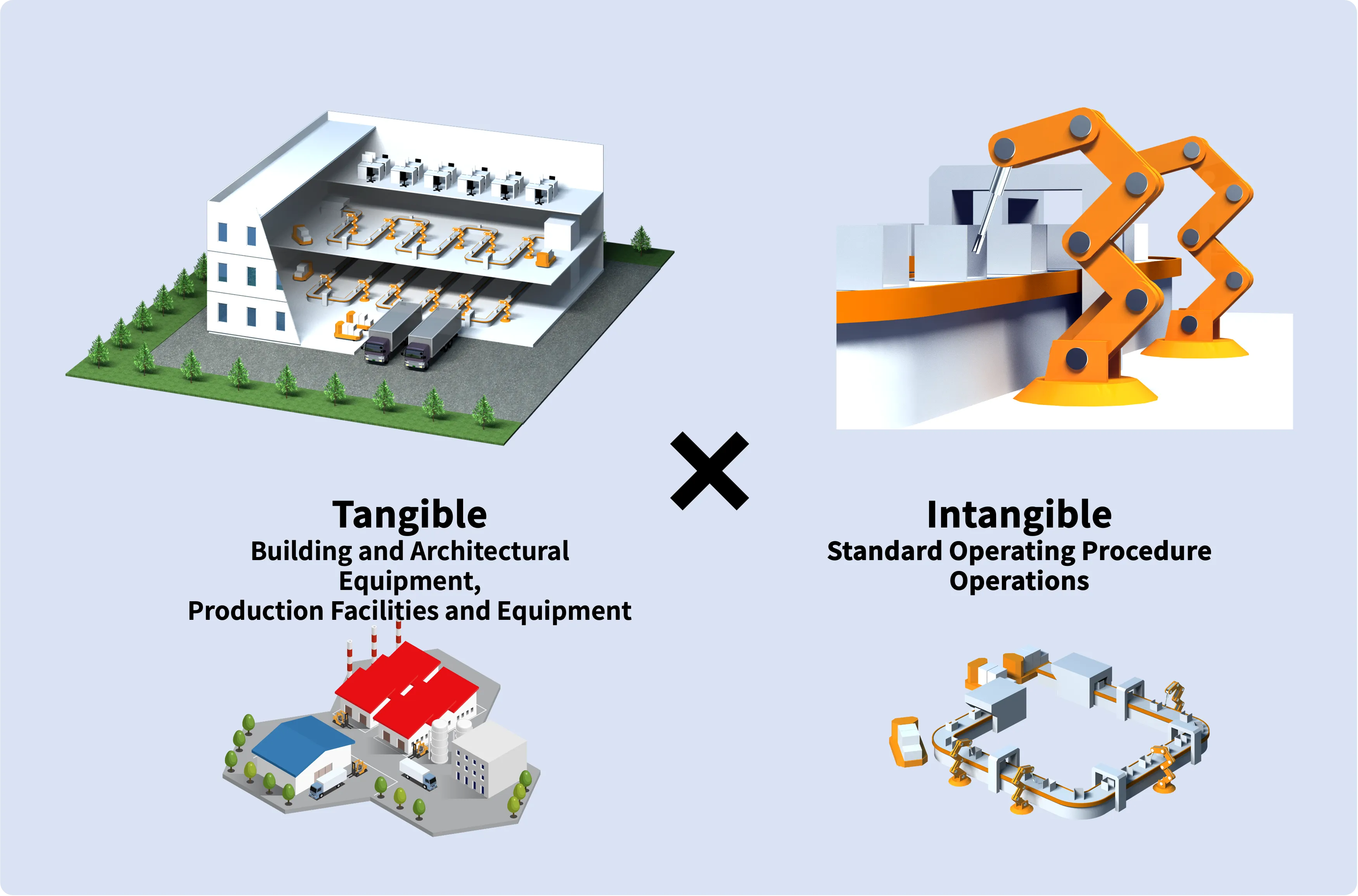
Our team consists of project managers and professional engineers experienced in factory construction across various fields. This allows us to manage the overall project schedule comprehensively, including not only the building and production support facilities but also providing support for the procurement, production management, and coordination of delivery plans of production equipment.

Services provided by CM Plus

Business Concept
We listen to our clients’ requirements and create deliverables such as a list of key equipment, PFD (Process Flow Diagrams), and conceptual layouts to visualize the investment image. Based on the created deliverables, we calculate an approximate investment amount to support investment decisions (Feasibility Study, F/S).

Conceptual Design
In the basic planning (concept design) phase, we compile the client’s requirements and requests through discussions and document them as a User Requirement Brief (URB). We also calculate an approximate project cost based on the contents of the URB.

Basic Design
In the basic design phase, based on the contents of the URB, we create the necessary drawings and specifications for effective solicitation. Specifically, in the process design, we produce PFDs, P&IDs (Piping and Instrumentation Diagrams), data sheets, time schedules (OTS), and equipment specifications required for plant design. We also organize regulations and standards related to facility design and construction, government applications, and environmental conditions, support setting up the project’s master schedule and overall execution plan, and assist with preparations and support for prior coordination with relevant government agencies and utility providers.

Procurement
In the purocurement phase, to build an optimal execution system tailored to the project’s characteristics, we study the order and service divisions and prepare solicitation specifications and procurement specifications based on these considerations. We then solicit quotations from construction companies. We support the client in selecting a construction company by conducting technical evaluations and quotation assessments on the proposals presented by the construction companies and summarizing them in a report.

Detailed Design / Production Management
In the detailed design and manufacturing management phase, we perform design supervision to ensure the construction company and production equipment suppliers reflect the URB, basic design documents, and various regulations in their designs and that progress is on schedule.

Construction and Installation Phase
In the construction and installation phase, we perform construction supervision to ensure that the construction company reflects the URB, URS, detailed design documents, and various regulations in their work, and manage the quality of facilities and equipment. Additionally, a construction manager will reside on-site to manage construction status, progress, and changes from the client’s perspective.

Trial operation
To carry out high-quality and efficient qualification, we provide services that suit the client’s needs, reducing their burden by supporting the creation of a validation master plan and risk assessment from the planning stage and creating protocols for Qualification (DQ, IQ, OQ, PQ), and supporting the execution phase.
Facility Operation
Related Projects
Related Contents
Useful Information
Please refer to the articles on our information dissemination site, “GMP Platform,” which is operated by our company.

“How to Build a Pharmaceutical Plant”
Aggregating expertise for the successful execution of pharmaceutical plant construction projects. From project structuring and basic planning to basic and detailed design, construction, and validation, we provide detailed explanations and key points for each step of the process.