In the previous issue, we discussed the overall roadmap for DX (Digital Transformation) and organized the control system hierarchy.
Table of Contents
Approach to DX Implementation
DX Implementation Approach
CM Plus proposes a DX implementation approach.
In order to ensure a solid digitalization process, the first step is to clarify what you want to do and organize your requirements. We will organize the main issues to be solved related to the project’s external environment, equipment, and production operations, the data collection methods needed to solve them, and the requirements for digitization and systemization of the data collection methods. Next, a list of candidate digital data and packaged systems and tools that contribute to digitization and smartening is compiled and prioritized in light of the above DX requirements. Then, based on the above, a basic concept of digitalization, digital technology, and IT/OT implementation will be developed based on the possible DX levels of digital data collection, sharing/visualization/analysis, and forecasting/learning/automation as indicated above.
Fig.1 Approach to DX Implementation
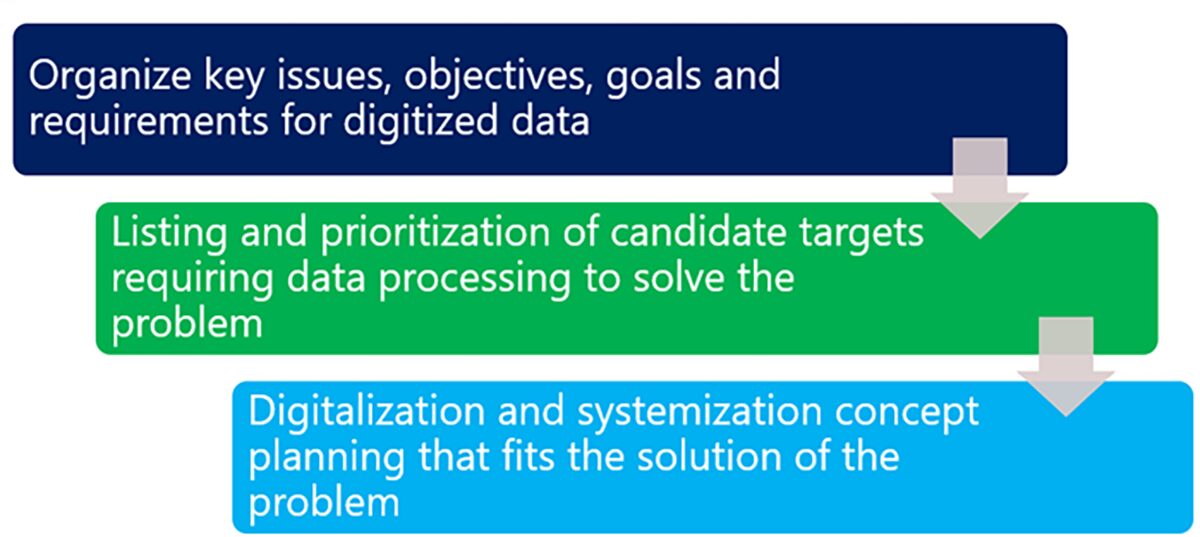
Clarification of the Issues
As stated in the previous Column Fig.2, DX on Digital Data (DX Progress Level – Expected Effectiveness), we believe it is most important to clarify what the goal is and the purpose of technology implementation.
Table 1: List of Technology Implementation Objectives
| Man (& Management) |
|
|---|---|
| Machine & Material |
|
| Method (& Managemant) |
|
Steps in Digital Data DX
We believe that it is possible to solve problems by taking the approach of implementing individual systems and tools to achieve the issues, objectives, and goals in each step of digital data collection, sharing/visualization/analysis, and prediction/learning/automation.
Fig.2 Steps in Digital Data DX (System Selection)
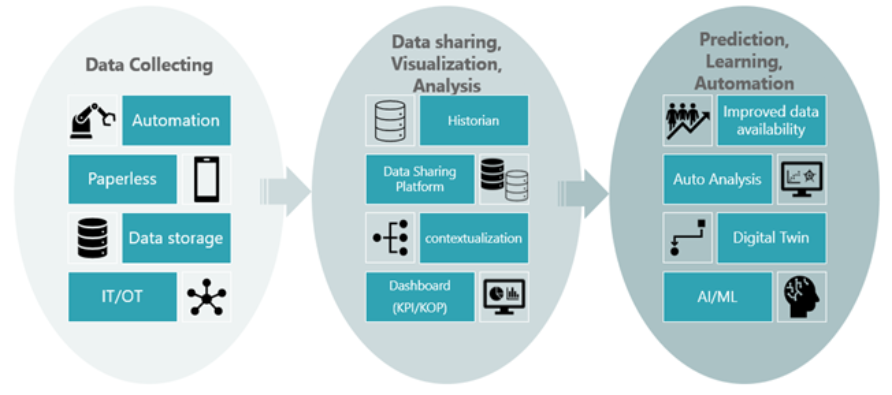
Al:Artificial Inteligence
ML:Machine Learning
Case Studies of Digital Technology Implementation
The following are examples of digital technology implementation cases in which we have been involved.
In each case, we have assisted in the creation of deliverables and the formulation of specifications at each stage by taking the following approaches: “Clarification of issues to be addressed,” “Survey and selection of available tools and systems,” and “Building-up the Overall system conception”.
We also published books and launched “IVEXL” internet platform, which we hope you will take a look at when you have a chance.
<Case Studies of our DX Support Services>
- Food Industry
→New Factory FS,CD Digital Technology Investigation, Conceptual Design - Polymeric Bulk Pharmaceutical Plant
→New Plant Basic Design Preparation of construction inquiry documents (ITOT outline and control specifications) - APIs Plant
→New Plant Basic Design Preparation of construction inquiry documents (control overview and system diagrams) - Low-molecular-weight bulk pharmaceutical
→New Plant Basic Design Preparation of construction inquiry documents (control system outline) - “How to Build a Pharmaceutical Plant”(to be published in July 2023)
→Partial control, referring to DX - Sponsored by “iVEXL”
→Providing a web-based digital exhibition platform for life sciences
FS:Feasibility Study
CD:Concept Design
BD:Basic Design
Steps of Digital Data DX
Based on the stages of technology implementation and DX realization levels described above, we propose the following steps for DX implementation in pharmaceutical plants: 1. In order to realize this one-stop solution, we need advanced engineering, project management, GMP consulting, and system integration capabilities for pharmaceutical plant construction projects. CM Plus has all of these capabilities. We are not a specialized system integrator, but rather participate in projects as an owner’s consultant, acting on behalf of the client. We have a team of PM, EM, CM, VM, GMP regulatory consulting, and GMP training for projects in the life science business.
Proposal from CM Plus Corp.

If you need help with DX for your pharmaceutical factory, please feel free to contact us.








