Iwata Label Co., Ltd.



Iwata Label Co., Ltd. has hired CM Plus Corporation to provide engineering services for its new plant construction project. In the following interview, Iwata Label’s CEO Masato Iwata and Aichi Plant Manager Takayuki Tsuji discuss how they came to choose CM Plus and how effective its services have turned out to be. (Interviewers: CM Plus Founder & Group CEO Tsunehiro Togashi and CM Plus Director Shigeru Nakamura.)
-

Iwata Label Co., Ltd.
Chairperson & CEO
Masato Iwata -

Iwata Label Co., Ltd.
Manager of Aichi Plant
Manufacturing Department
Takayuki Tsuji -

CM Plus Corporation
Founder & Group CEO
Tsunehiro Togashi -

CM Plus Corporation
Director
Shigeru Nakamura

From left:
Tsunehiro Togashi, Founder & Group CEO, CM Plus Corporation
Masato Iwata, Chairperson & CEO, Iwata Label Co., Ltd.
Takayuki Tsuji, Manager of Aichi Plant, Manufacturing Department, Iwata Label Co., Ltd.
Shigeru Nakamura, Director, CM Plus Corporation
1 Tight management of cost and schedule coupled with flexibility
Togashi: First, let me congratulate you on winning the prestigious Outstanding Corporate Manager Award, Mr. Iwata. I’ve been looking forward to talking with you, and of course with you too, Mr. Tsuji. I’d like to start by asking how you decided to hire an external consultant for your project.

Iwata:Our project to build a new manufacturing base was launched in April 2015, and the motto we chose for this venture was “By responding to radically shifting customer needs, we move 15 years ahead of our rivals.” Until then, we had been conducting manufacturing operations at an existing plant that had been repeatedly expanded since the company’s inception in 1962. Our manufacturing processes and quality control systems have steadily improved thanks to the advice of our longstanding pharmaceutical clients, and no major problems have been pointed out at quality audits in recent years. The facilities were aging, however, and we sensed that our customers were having doubts about the adequacy of some of our practices, such as insect control, although they didn’t say it in so many words. Also, considering Japan’s imminent participation in the Pharmaceutical Inspection Co-operation Scheme (PIC/S) and the likely growth of our overseas business, we felt we had to do things differently for the ongoing renovation project. That is why we decided to hire an external consulting firm with expertise in the pharmaceutical manufacturing business.
How did you go about looking for a consultant, and what were the points that were in favor of CM Plus as a business partner?
Tsuji::Our CEO, Iwata, was referred to you by a former top executive of one of our long-term clients. We then informed you about the concept and prospective size of the new plant and invited you to tour the existing plant so you could draw up a concrete proposal. We similarly approached other consulting firms. The factors that worked in favor of CM Plus were its solid track record in the pharmaceutical sector, the emphasis on strict cost and schedule control, and the flexibility to choose a plan from several alternatives. Also, none of our project members had any experience in constructing a new plant, so your expertise in this respect was very reassuring—which was also the key.
Togashi: I’m glad you noticed how useful we could be to you. In fact, we make it a rule to control the schedule, cost, and quality from the standpoint of our clients because that is essential to the success of any project.
2 Integrated services that cover everything from conceptual design to construction
Togashi: Have our services met your expectations?
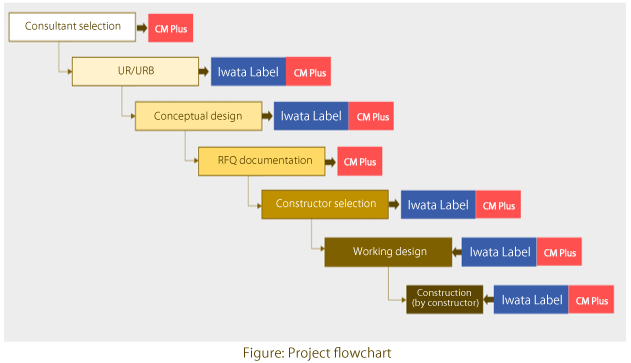
Iwata: CM Plus has been providing us with integrated project management services that cover everything from conceptual design and constructor selection to actual construction. We have already chosen a constructor and are now developing a working design. During the conceptual design phase, we were able to picture specific images of the HVAC systems and building structures we would need, thanks to the pharmaceutical production know-how provided by CM Plus. Without developing a conceptual design, we would not have been able to sort out the requirements or prepare the RFQ documentation. During the screening of constructors, CM Plus offered us expert help in scrutinizing the candidates’ quotes, and we are thankful that we could choose the best applicant. We are now developing a working design. Under strict control of costs and schedules—which is exactly what we expect from CM Plus—we have already held several fruitful meetings with the chosen constructor. So far, we are quite satisfied.

Togashi: Our motto is to provide just the right amount of services when and where they are needed. We want to help our clients implement their entire projects—from planning and design to construction—quickly, inexpensively, and with good quality. I’m pleased to learn both of you rate our services favorably.
Iwata: When we built the current plant in Tochigi 20 years ago, we had some issues with specifications and pricing that affected us negatively long afterwards. Because of this bitter experience, we felt we should seek the help of outside experts. And we are glad we did approach you.

Nakamura:We hope you will become our repeat customer! Is there anything that could further improve our services?

Tsuji:We definitely want you to oversee this project to completion. I can only think of one thing that needs improvement. Since our headquarters are located far apart, holding meetings with you requires either party to make a trip, which takes up precious time. We sometimes teleconference over the web to address this issue, but the connection has often been unstable. If this issue is resolved, we’d give you an even better appraisal.
Togashi: I’m confident that our IT systems are as good as those of any top-tier corporation, but we are aware of the web conferencing issues and intend to rectify the glitches as soon as possible.
3 A center for further growth of Iwata Label
Togashi: Please tell us about the roles you expect us to play going forward.
Tsuji: As the construction phase starts, we will no longer be discussing the concepts but will be monitoring the actual progress of the work on-site. As I mentioned before, our project team members have little experience supervising construction work, so we are unsure if we can correctly assess the situation. We’d like CM Plus to continue controlling the costs and schedules as before and also to perform construction management as thoroughly as you can to ensure the construction is done as designed. Once the facilities are in place and operations begin, our customers will pay visits and conduct audits. We hope CM Plus will advise us on how to deal with audits most efficiently.
Togashi: Speaking of audits, our expertise covers three areas, including engineering, GMP consulting, and business matching. That means our services to you can be extended from consulting to engineering or from engineering to consulting and so forth. We hope we can satisfy your needs more fully through our integrated service offerings. Lastly, we’d like to hear about your particular strengths and how you plan to expand your business going forward.
Iwata:As a specialized manufacturer of pharmaceutical labels and labeling machines (which we call “labelers”), we enjoy the patronage of many pharmaceutical firms. Our labels can be much more than just a means to identify what’s in a container. They offer diverse functions designed to prevent damage and human error and ensure safety in medical settings, and our labelers automate the production of our unique labels. Armed with these products, we are now expanding our business overseas. With the aim of remaining an indispensable player in the pharmaceutical sector, we are constructing a new plant with CM Plus, which we are calling the Innovation Park. We plan to attain continued growth though this strategic center for product development and manufacturing.
Togashi: CM Plus offers diverse services in the field of pharmaceuticals. Regarding the labelers you’ve developed, for instance, we would be happy to advise you on such matters as computer system validation (CSV) and qualification based on the latest know-how at our command.
- Established:
- 1962/10/22
- Business activities:
- Manufacturing and sale of tack labels and labeling machines Manufacturing and sale of stickers Printing and processing of packaging materials; manufacturing and sale of compact printing presses Manufacturing and sale of other equipment related to packaging
- Headquarters:
- 5-15-18 Mitsui, Ichinomiya-shi, Aichi 491-0827 Japan
- Representative:
- Masato Iwata, Chairperson & CEO

Vial Protect Pack®Ⅰ
Vial Protect Pack®Ⅱ

Press-through package (PTP) labels
(Large print labels, medication assistance, preventing accidental drug intake by children)

Labeling machines
Vial protect pack labeler YOROI
Preparation of conceptual design and construction RFQ, support for constructor selection
Management of basic and detailed designs
Construction management
* The information contained in this article is those at the time of the interview.